[ad_1]
Atmospheric acidity is increasingly established by organic acids, such as formic acid and carbon dioxide. The former contributes to the development of aerosol particles as a precursor to raindrops and thus affects the pH of rainwater and cloud growth..
In previous models of the atmospheric chemistry of acid formation, formic acid had played an insignificant role. The chemical processes involved in its formation were not fully understood.
Under the aegis of the Forschungszentrum Jülich, an international research team has now succeeded in filling this gap and decoding the dominant mechanism of the development of formic acid. This further improves atmospheric and climatic models. The results of the study were recently published in the peer-reviewed journal Nature.
The Germans are familiar with acid rain, especially from their experience in the 1980s. The reason for acid rain was that the sulfur oxides and nitrogen oxides released into the air by humans form acid. nitric and sulfuric acid by reacting with water droplets in clouds. The pH of acid rain is around 4.2 to 4.8, which is lower than that of pure rainwater which has a pH of 5.5 to 5.7. Normal acidity is due to the natural carbon dioxide content of the air.
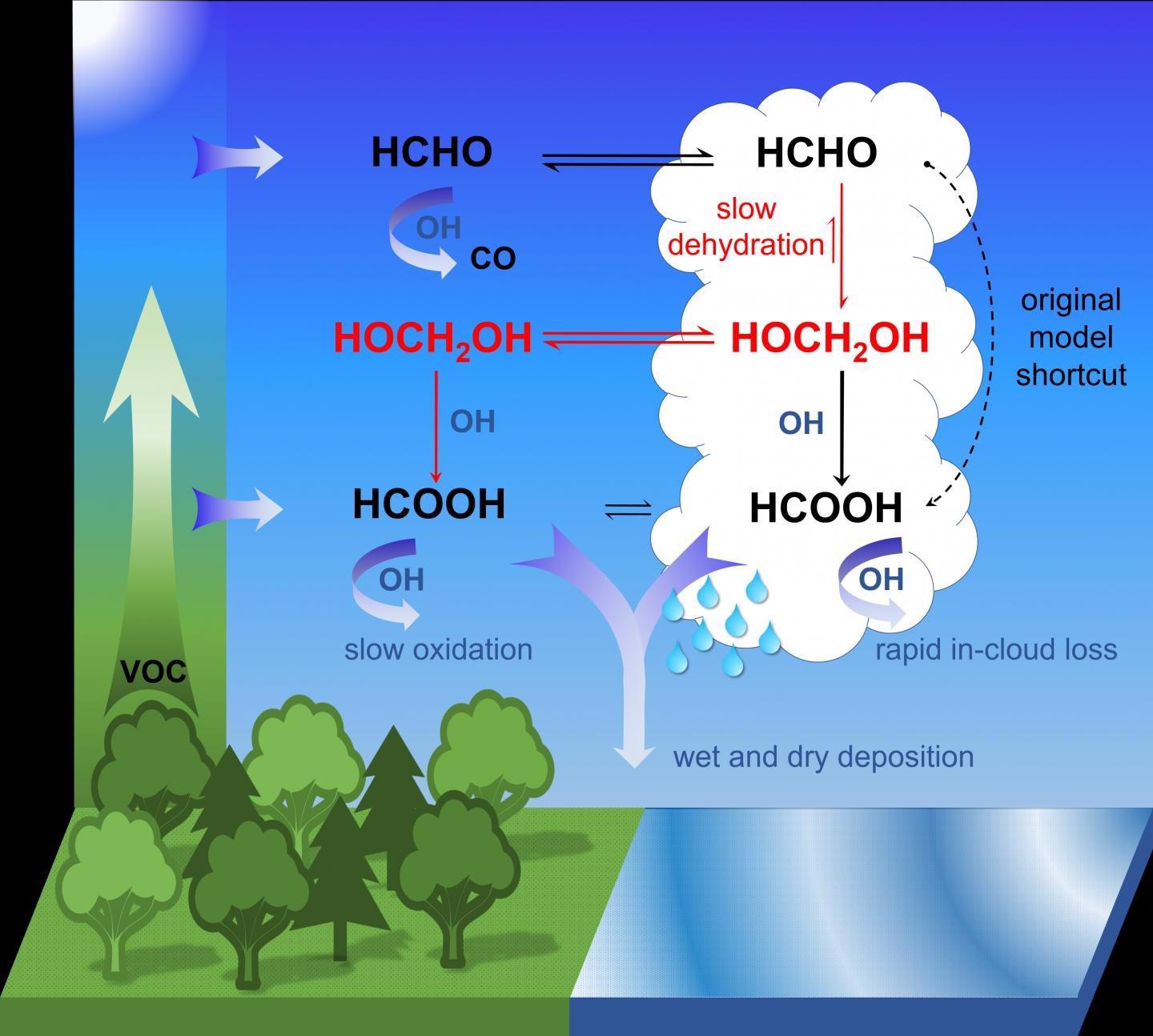
But the chemical process that takes place in most of the formic acid found in the air was not known until now. Dr Bruno Franco and Dr Domenico Taraborrelli from the Jülich Energy and Climate Research Institute – Troposphere have now successfully decoded it: formaldehyde is naturally formed by the photo-oxidation of volatile organic compounds.
Methanediol is formed when formaldehyde reacts in cloud droplets with water molecules. Most of this methanediol is released and then reacts with OH radicals, sometimes called “atmospheric detergents”, to form formic acid by a photochemical process. A smaller part also forms formic acid by reacting with the liquid phase of the water droplets and this acid is dispersed by rain.
According to our calculations, the oxidation of methanediol in the gas phase produces up to four times more formic acid than what is produced in other known chemical processes in the atmosphere..
Domenico Taraborrelli, Energy and Climate Research Institute, Forschungszentrum Jülich
This amount lowers the pH of rainwater and clouds to 0.3, which underlines the contribution of organic carbon to the natural acidity present in the air.
Initially, the two researchers tested their concept using MESSy, a global model of atmospheric chemistry, and compared the results with remote sensing data. To perform the modeling, the team used the Jülich JURECA supercomputer. The results were confirmed by subsequent experiments carried out in the SAPHIR atmosphere simulation chamber at Jülich.
We assume that the demonstrated mechanism is also active in aqueous aerosols and applies to other organic acids such as oxalic acid, which are not sufficiently taken into account in atmospheric chemistry models to date..
Domenico Taraborrelli, Energy and Climate Research Institute, Forschungszentrum Jülich
One of the impacts of this could be a better understanding of aerosol particle growth and cloud formation.
Journal reference:
Franco, B., et al. (2021) Pervasive atmospheric production of organic acids mediated by cloud droplets. Nature. doi.org/10.1038/s41586-021-03462-x.
Source: https://Www.Fz-Juelich.De/
[ad_2]